Scans reveal Dolly and her contemporaries had aged normally
Concerns that cloning caused early-onset osteoarthritis (OA) in Dolly the sheep were unfounded, a new study has concluded.
Findings published in the journal Scientific Reports show that Dolly and her contemporaries had aged normally with no clinical signs of OA. Researchers found radiographic evidence of only mild, or in one case, moderate OA.
The results appear to be in stark contrast to reports that led to much debate over the possibility of early-onset disease in cloned animals.
Professor Kevin Sinclair, one of the team members that carried out the research, said: “Our findings of last year appeared to be at odds with original concerns surrounding the nature and extent of osteoarthritis in Dolly – who was perceived to have aged prematurely.
“Yet no formal, comprehensive assessment of osteoarthritis in Dolly was ever undertaken. We, therefore, felt it necessary to set the record straight.”
Dolly was one of the first animals to be cloned from adult cells, attracting considerable scientific and media attention across the world. In 2003, reports emerged that Dolly was suffering from osteoarthritis at the age of five - a disease which is normally found in older sheep.
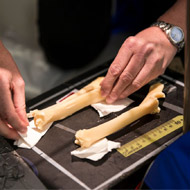
The only formal record of OA in Dolly is a brief mention in an abstract report. In absence of the original clinical records, a team of researchers from the Universities of Nottingham and Glasgow set out to see whether the concerns were justified.
They travelled to Edinburgh, where the skeletons of Dolly, Bonnie (her naturally-conceived daughter) and Megan and Morag (the first two animals to be cloned from differentiated cells) are kept. They performed a radiographic assessment on the skeletons and then compared them with x-rays of sheep that had been naturally conceived.
“We found that the prevalence and distribution of radiographic-OA was similar to that observed in naturally conceived sheep, and our healthy aged cloned sheep,” said Professor Sandra Corr, who was part of the investigation team. “As a result, we conclude that the original concerns that cloning had caused early-onset OA in Dolly were unfounded.”
Image (C) University of Nottingham.



 FIVP has shared a survey, inviting those working in independent practice to share their views on the CMA's proposed remedies.
FIVP has shared a survey, inviting those working in independent practice to share their views on the CMA's proposed remedies.