Stem cell therapy treats chronic oral disease in cats
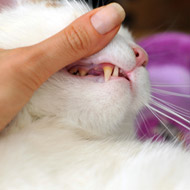
Vets used stem cell therapy to cure cats suffering from feline chronic gingivostomatitis.
A novel treatment has been used in the US to treat cats suffering from a painful oral disease.
In a clinical trial, veterinary dental surgeons at the the UC Davis School of Veterinary Medicine used stem cell therapy to cure cats suffering from feline chronic gingivostomatitis (FCGS).
The study is the first of its kind to prove the safety and efficacy of using stem cell therapy for the treatment of the condition.
Vets first became interested in running the trial after seeing several cases where cats had their teeth removed to treat FGCS.
Despite following up the removal with several courses of corticosteroids and antibiotics, the cats still experienced a great deal of pain and suffering.
“FCGS is a challenging disease to treat, and we were frustrated that some cats wouldn’t respond to traditional treatment,” said Boaz Arzi, lead author and veterinary dental surgeon at UC Davis. “We were banging our heads against the wall and this stem cell therapy was a last resort.”
The technique involved taking cat’s own fat-derived stem cells, processing and characterising them. The cells were then given back to the cats intravenously to reduce inflammation and promote tissue regeneration.
Scientists hope that FCGS also has the potential to serve as a useful model for the treatment of oral inflammatory disease in humans. Nasim Fazel, a dentist at the UC Davis Health system has been working with the veterinary team to perform comparative studies.
“I was really excited to hear about their work because the cat disease behaved very similarly to what i saw in my human patients, said Fazel.
Based in the success of this research, she recently submitted a grant to establish a human clinical trial to treat oral lichen plans - a similar chronic inflammatory disease in humans.
“We’re in desperate need of novel therapies to treat chronic inflammatory mucosal disorders such as OLP, which are challenging to treat and of major impact to patients’ quality of life,” Fazel said.
“Having this opportunity to translate what we’re learning in veterinary medicine to human medicine and working together to bring therapies discovered in the cat model to chronic oral inflammatory diseases in humans is exciting and has great potential.”
Results of the trial recently appeared the journal Stem Cells Translational Medicine.



 FIVP has shared a survey, inviting those working in independent practice to share their views on the CMA's proposed remedies.
FIVP has shared a survey, inviting those working in independent practice to share their views on the CMA's proposed remedies.